AB Cube SafetyEasy®
•Drive Phase PV offers safety data management solutions with AB Cube’s SafetyEasy Database - a cloud-based database software for effortless pharmacovigilance (PV) compliance, but also for medical device (MD) vigilance, cosmetovigilance and nutrivigilance
•Streamlined system for processing and reporting adverse events in a fully E2B(R3) compliant manner
•Quick to set up with implementation within 2 weeks, and can be uniquely configured according to the client’s needs
•In 2021, SafetyEasy was selected by the Agence nationale de sécurité du médicament et des produits de santé (ANSM) as its PV safety database.
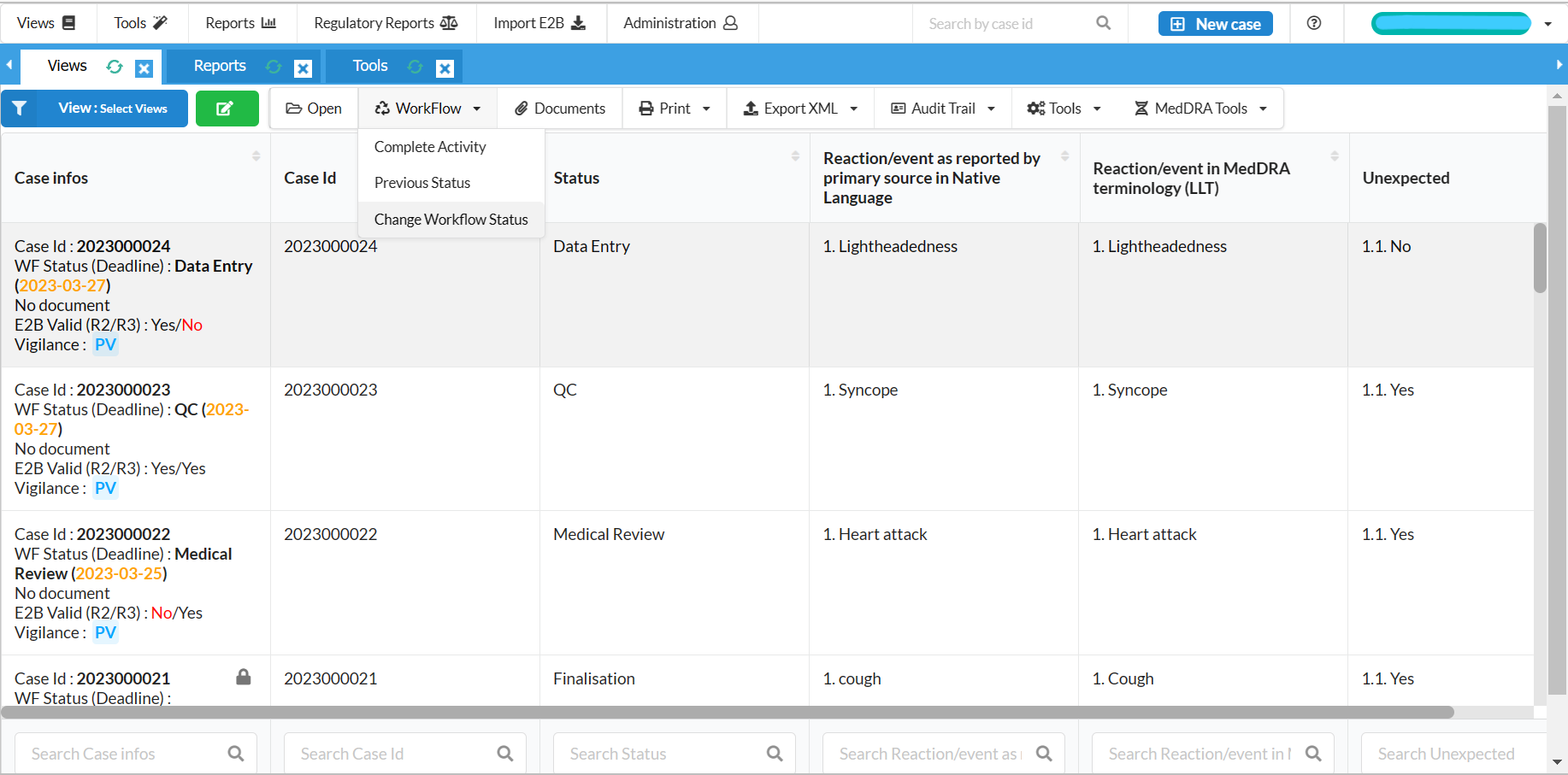
Case Processing
Full activity workflow management
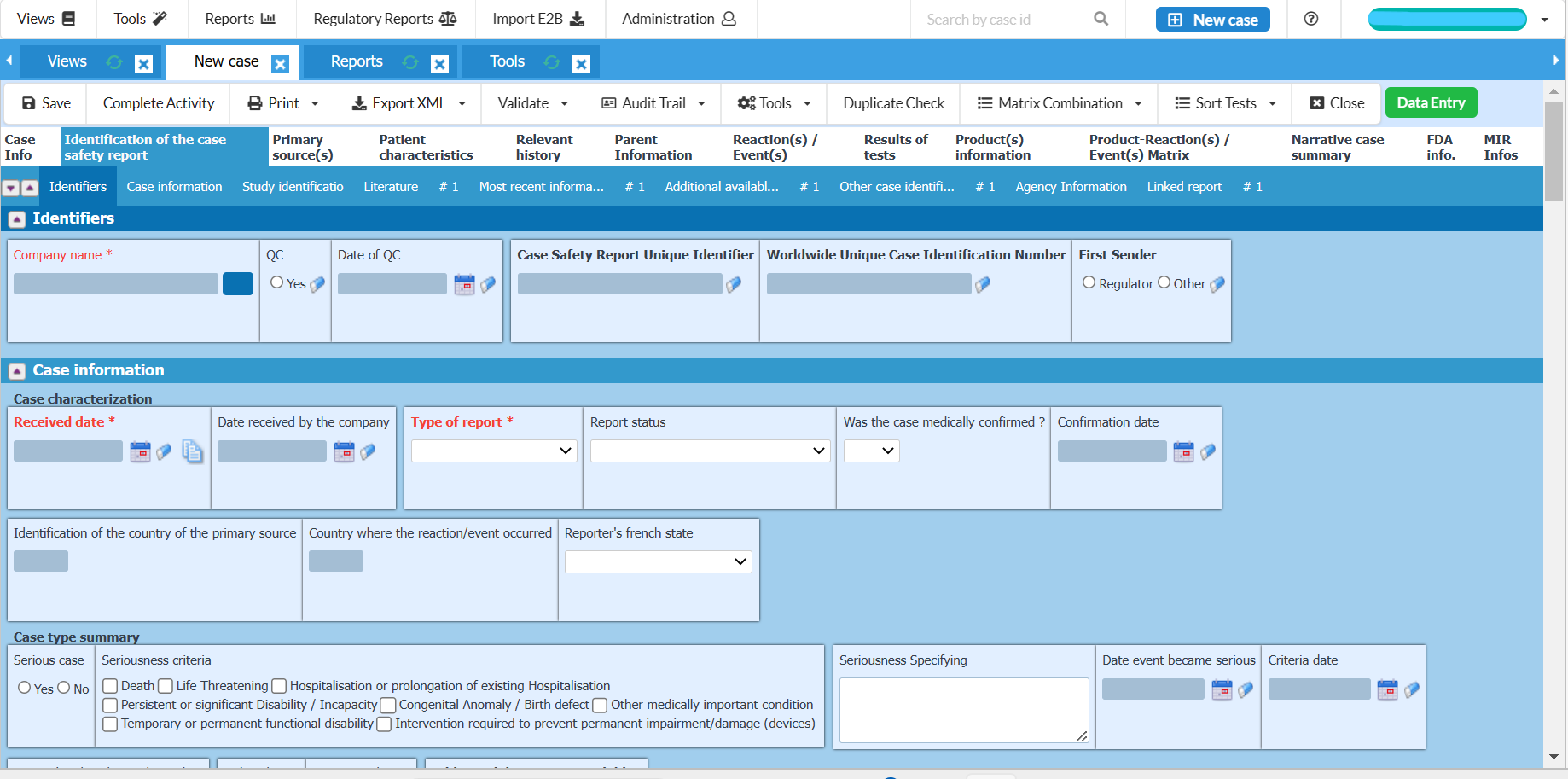
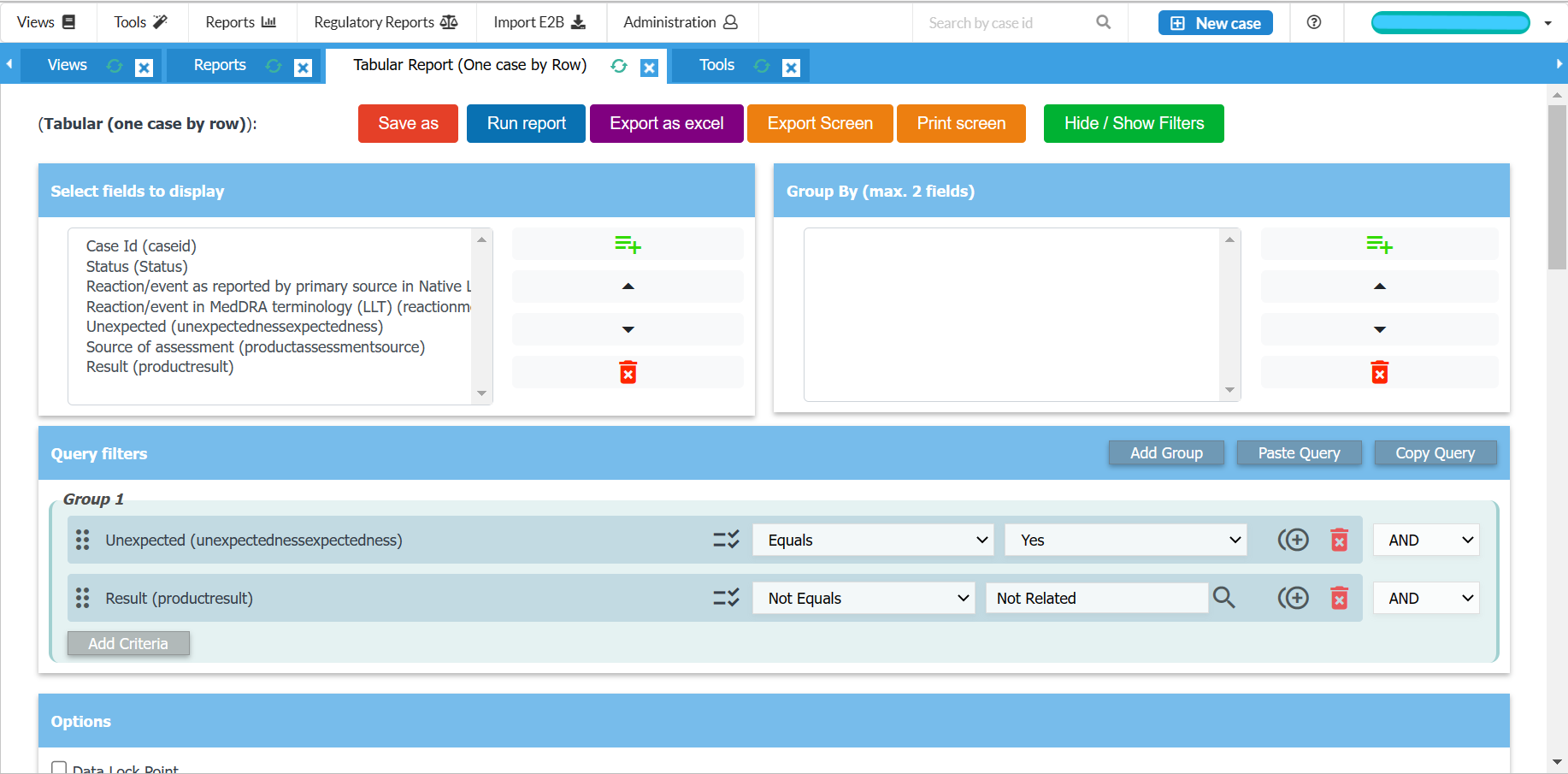
Easy to use data entry system with E2B (R2/R3) fields
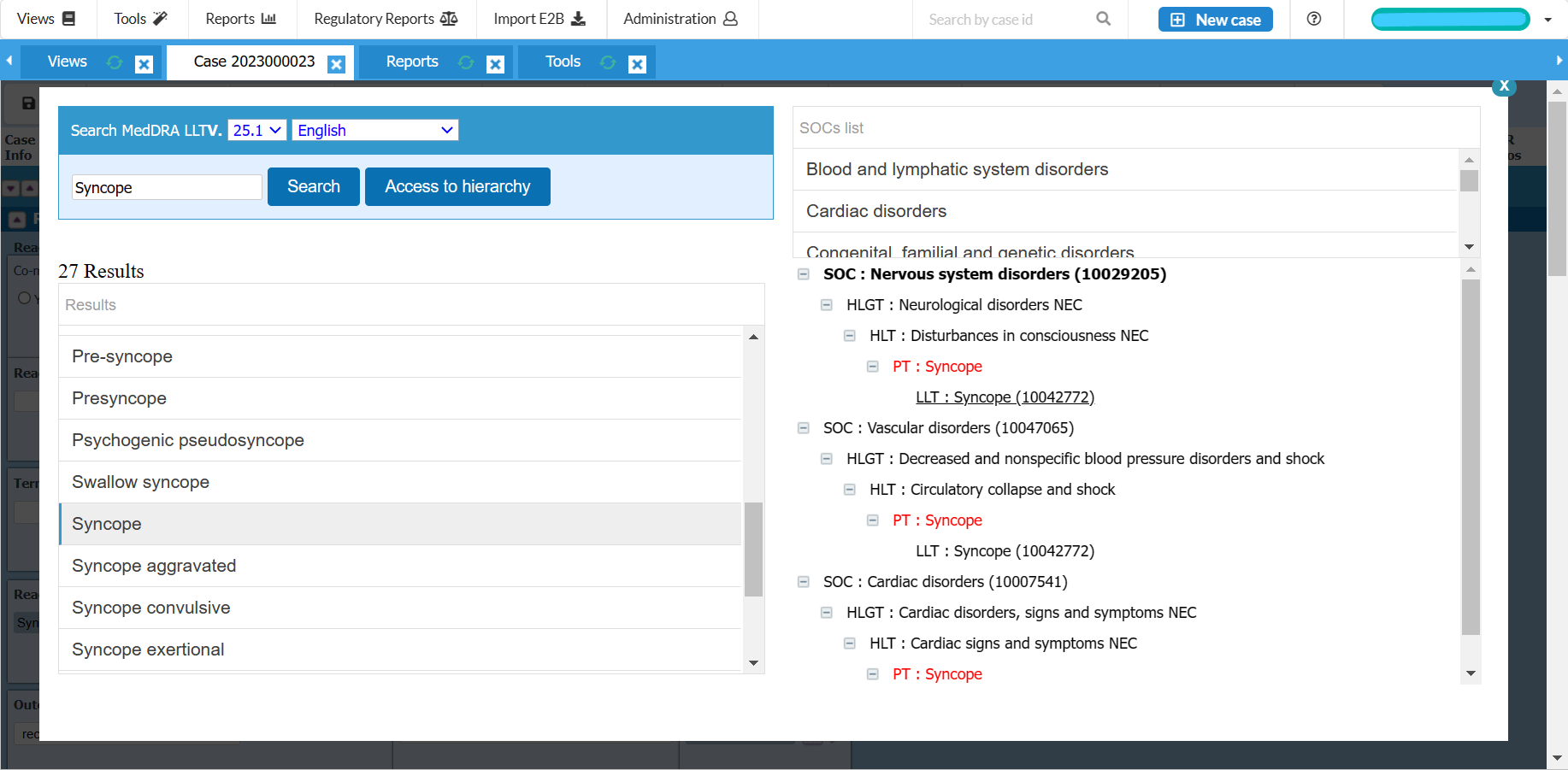
Inbuilt MedDRA Coding Dictionary and WHO Drug Dictionary integration
XEVMPD IMPD dictionary integration and management
Report Generation
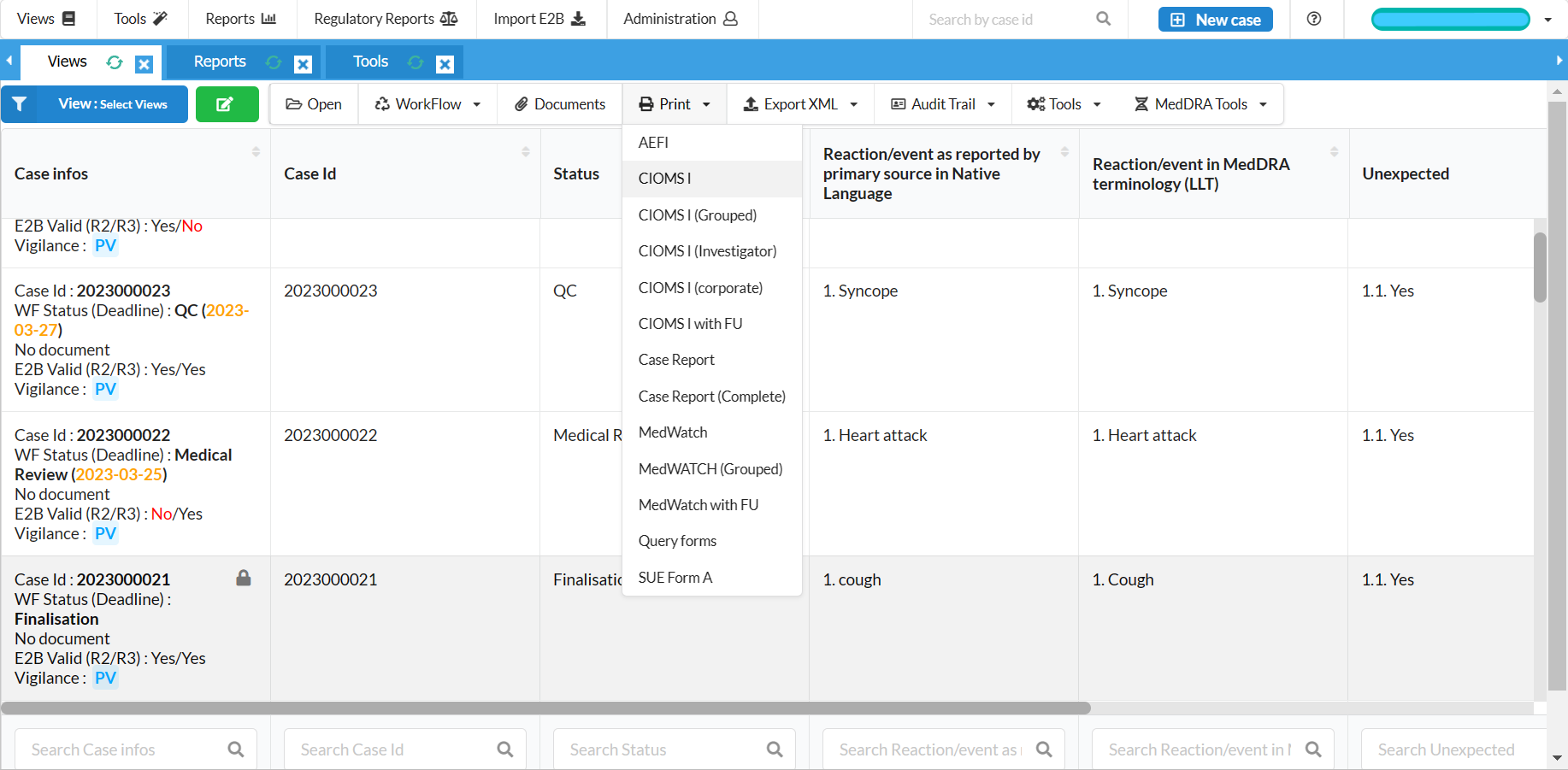
Generate CIOMS I, MedWatch, XML reports
Generate line listings and summary tabulations for DSURs, PSURs, PBRERs
Export tabulations to Microsoft Excel
Perform signal detection analysis (PRR, ROR, qualitative)
Regulatory Requirements
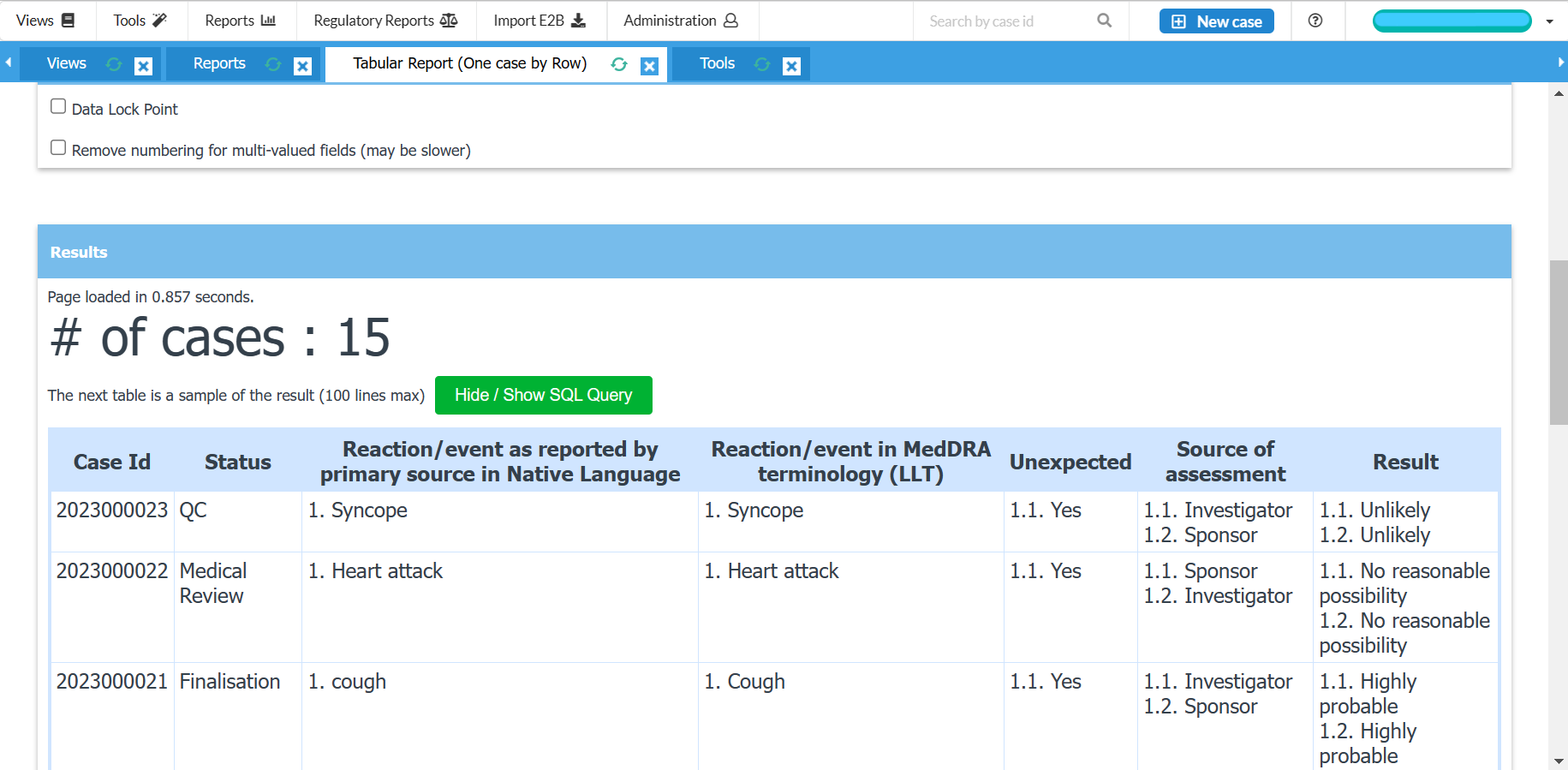
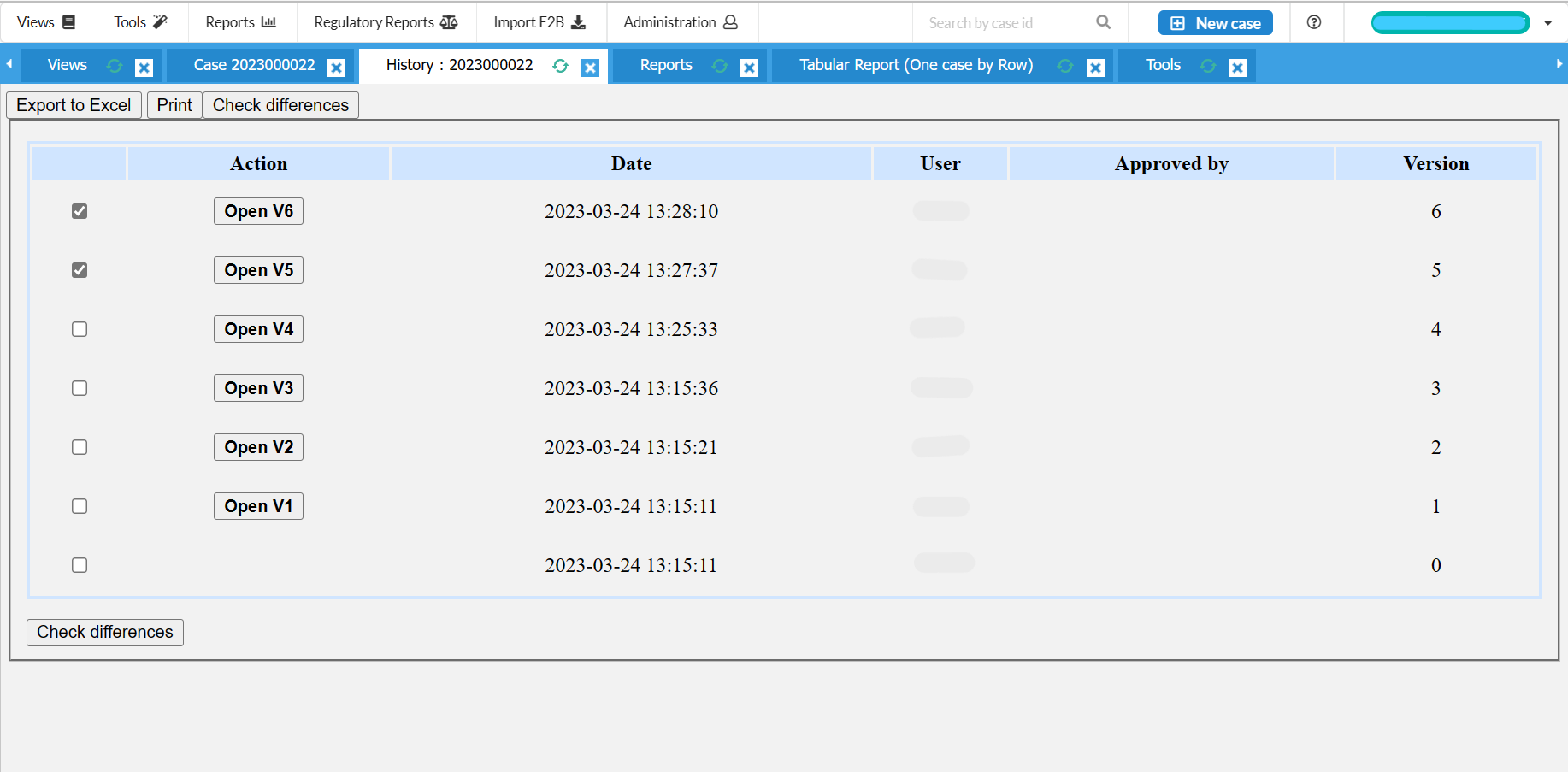
Full case history, audit trail and reporting summary for cases
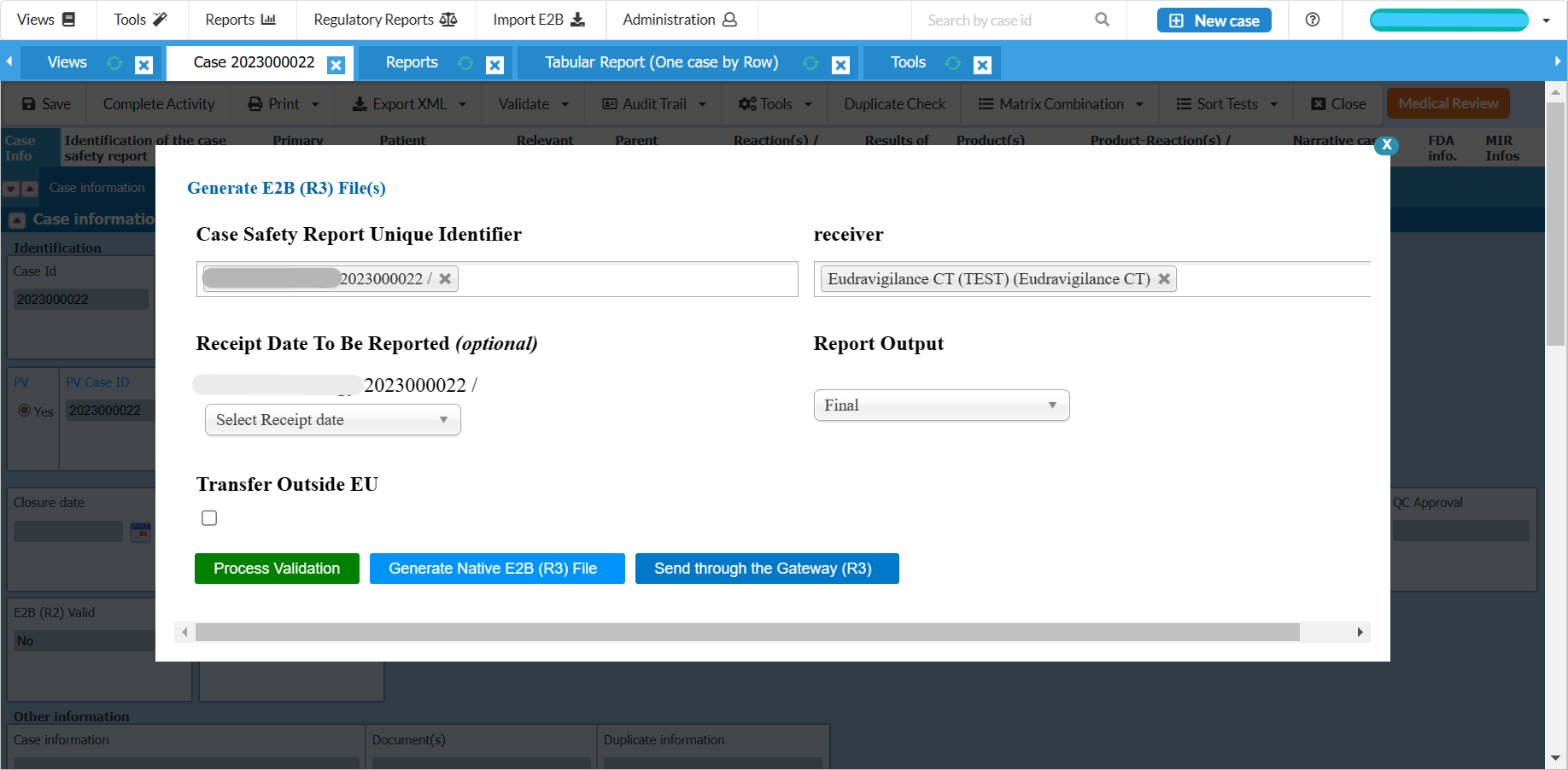
Fully E2B(R3) compliant and gateway compatible
Conforms to the latest FDA, EMA and MHRA requirements
All vigilance solutions are validated according to GAMP 5 and are compliant with HIPAA, FDA 21 CFR Part 11, ISO 27001 Data Center
Although Drive Phase offers the SafetyEasy Database as a standard for safety data management, our team are also proficient in using database systems such as ARISg, Argus, TARA PV (www.tarapv.com) and Veeva Vault Safety.